First images of an ‘upgraded’ CRISPR tool captured
Posted: 20 December 2019 | Drug Target Review | No comments yet
Researchers have utilised cryo-electron microscopy and used the images they captured with an electron microscope to generate atomic resolution models of the INTEGRATE system.


The first images of a new gene-editing tool that could improve upon existing CRISPR-based tools have been captured by researchers at Columbia University.
The team developed the tool, called INTEGRATE, after discovering a unique ‘jumping gene’ in Vibrio cholerae bacteria that could insert large genetic payloads in the genome without introducing DNA breaks. They then harnessed a technique called cryo-electron microscopy to freeze the gene-editing complex in action, revealing high-resolution details about how it works.
“We showed in our first study how to leverage INTEGRATE for targeted DNA insertions in bacterial cells,” says Sam Sternberg, PhD, Assistant Professor of Biochemistry and Molecular Biophysics at Columbia University Vagelos College of Physicians and Surgeons, who led the research with Israel Fernandez, PhD, assistant professor of biochemistry & molecular biophysics at Columbia. “These new images, a wonderful collaboration with Israel Fernández’s lab, explain the biology with incredible molecular detail and will help us improve the system by guiding protein engineering efforts.”
The researchers used cryo-electron microscopy, which involves flash-freezing a sample of the gene-editing complex in liquid nitrogen and bombarding it with electrons. They then used the images they captured with the electron microscope to generate atomic resolution models of the INTEGRATE system.
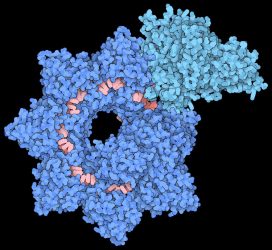

Structure of the INTEGRATE complex showing Cascade (dark blue), TniQ units (light blue), and guide RNA (red). Credit: Sternberg and Fernández Labs at Columbia University Irving Medical Center.
The structural model reveals that the complex is made up of two main sections that are arranged in a helical filament. The larger portion, called Cascade, winds around and carries a guide RNA that it uses to scan the cell for a matching sequence in DNA. Once it locates and binds the target sequence, it threads the DNA strand through the TniQ ‘transposition’ proteins that sit on the end of the complex and recruit other enzymes that help modify the DNA.
The scanning mechanism of INTEGRATE appears to work in a similar way to other well-studied CRISPR systems, some of which also contain a Cascade complex with guide RNA. However, unlike other CRISPR systems that use Cascade to target DNA for cutting, the function of Cascade within INTEGRATE is to target DNA for highly accurate insertion of genetic payloads.
In addition to informing future engineering efforts, the structures highlight a possible proofreading checkpoint, say the researchers. Existing CRISPR technologies can suffer from so-called ‘off-target effects’ in which unintended sequences are promiscuously modified. The researchers explain that the new structures reveal how Cascade and TniQ work together to ensure that only the correct ‘on-target’ sequences are marked for DNA insertion.
The researchers also say they plan to further explore this checkpoint while developing the tool for new therapeutic approaches to disease.
The study was published in Nature.
Related topics
CRISPR, DNA, Enzymes, Genetic Analysis, Imaging
Related organisations
Columbia University
Related people
Sam Sternberg PhD