Novel COVID-19 vaccine shows efficacy in mice
Posted: 3 April 2020 | Victoria Rees (Drug Target Review) | No comments yet
Researchers have developed a vaccine against the COVID-19 coronavirus which induced the rapid production of antibodies in mice.

The researchers use US Food and Drug Administration Good Manufacturing Practice to produce vaccines suitable for human clinical trials [credit: UPMC].
A potential vaccine has been developed to combat SARS-CoV-2, the coronavirus causing the COVID-19 pandemic. Researchers at the University of Pittsburgh School of Medicine, US, say that their new vaccine has shown efficacy when tested in mice.
Called the Pittsburgh Coronavirus Vaccine (PittCoVacc), the vaccine is delivered through a fingertip-sized patch and induces the production of antibodies specific to SARS-CoV-2 at quantities thought to be sufficient for neutralising the virus.
“We had previous experience on SARS-CoV in 2003 and MERS-CoV in 2014. These two viruses, which are closely related to SARS-CoV-2, teach us that a particular protein, called a Spike (S) protein, is important for inducing immunity against the virus. We knew exactly where to fight this new virus,” said co-senior author Dr Andrea Gambotto, associate professor of surgery at the Pitt School of Medicine. “That’s why it’s important to fund vaccine research. You never know where the next pandemic will come from.”
Compared to the experimental mRNA vaccine candidate that has just entered clinical trials, PittCoVacc follows a more established approach, using lab-made pieces of viral protein to build immunity.
When tested in mice, PittCoVacc generated a surge of antibodies against SARS-CoV-2 within two weeks of the microneedle prick.
Although the animal models have not been tracked over a long period of time, the researchers point out that mice who received their MERS-CoV vaccine produced a sufficient level of antibodies to neutralise the virus for at least a year and so far the antibody levels of the SARS-CoV-2 vaccinated animals seem to be following the same trend.
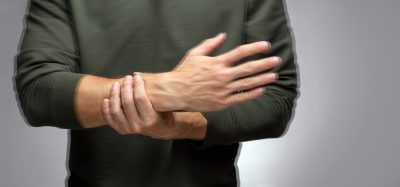
The researchers also used a novel approach to deliver the drug, called a microneedle array, to increase potency. This array is a fingertip-sized patch of 400 tiny needles that delivers the S protein pieces into the skin, where the immune reaction is strongest. The patch is applied similarly to a plaster and then the needles, made entirely of sugar and the protein pieces, dissolve into the skin.


The vaccine is delivered into the skin through a fingertip-sized patch of microscopic needles [credit: UPMC].
According to the vaccine developers, the system is also highly scalable. The protein pieces are manufactured by a ‘cell factory’ – multiple layers of cultured cells engineered to express the SARS-CoV-2 S protein – that can be stacked further to multiply yield. Purifying the protein can be conducted at industrial scale, say the team.
The researchers emphasise that the SARS-CoV-2 microneedle vaccine maintains its potency even after being thoroughly sterilised with gamma radiation – a key step toward making a product that’s suitable for use in humans.
“Testing in patients would typically require at least a year and probably longer,” said co-senior author Dr Louis Falo, professor and chair of dermatology at Pitt’s School of Medicine and UPMC. “This particular situation is different from anything we’ve ever seen, so we don’t know how long the clinical development process will take. Recently announced revisions to the normal processes suggest we may be able to advance this faster.”
The paper appears in EBioMedicine, which is published by The Lancet.
Related topics
Antibodies, Biopharmaceuticals, Drug Development, Immunology, Research & Development, Targets, Vaccine
Related conditions
Coronavirus, Covid-19
Related organisations
University of Pittsburgh School of Medicine
Related people
Dr Andrea Gambotto, Dr Louis Falo