New malaria vaccine candidate shows promise in non-human primates
Posted: 24 April 2020 | Victoria Rees (Drug Target Review) | No comments yet
Using an antibody to a particular malaria protein called PfGARP, researchers have developed a new strategy for designing a malaria vaccine.

Researchers at Brown University, US, say they have discovered a promising novel strategy for combatting malaria, presenting a new vaccine candidate for the condition.
The researchers screened blood samples from children who had natural immune resistance to severe malaria infection. The study identified an antibody to a particular malaria protein, called PfGARP, that appears to protect resistant children from severe disease. Lab tests showed that antibodies to PfGARP seem to activate a malarial self-destruct mechanism, causing parasite cells living inside human red blood cells to undergo a form of programmed cell death.
The team is hopeful that vaccinating individuals with PfGARP to generate anti-PfGARP antibodies, or directly infusing anti-PfGARP antibodies, would protect them against severe malaria. The researchers developed preliminary versions of those vaccines and testing in non-human primates has shown promise, the researchers report.
“We demonstrated in two independent studies in non-human primates that vaccination with PfGARP protects against a lethal malaria parasite,” said study senior author Dr Jonathan Kurtis, a professor at the Warren Alpert Medical School of Brown University. “What’s exciting is that this is a vaccination strategy that attacks malaria in a way that it has never been attacked before – one in which the parasite becomes complicit in its own demise. We are hopeful that this vaccine, perhaps combined with other malarial antigens, will translate into a strategy that can help prevent severe malaria in people.”
The beginning of the research
In 2001, epidemiological research led by Michal Fried and Patrick Duffy of the US National Institutes of Health (NIH) began recruiting cohorts of children in Tanzania. The children were enrolled at birth and followed for years to see who among them developed an acquired immune response to malaria.
“There was a ton of hard epidemiological work that went into simply identifying which children were resistant and which were not,” Kurtis said. “Only after we knew their resistance levels could we use this information to identify the parasite targets that were recognised by antibodies made only by the resistant children but not by the susceptible children.”
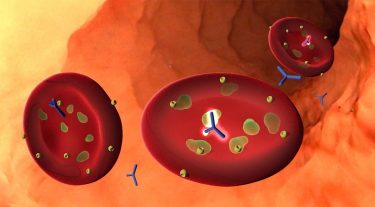

A new study identifies an antibody, often present in children with natural immunity to severe malaria, that attacks a particular malaria protein called PfGARP. When the antibody binds the protein, it causes malaria cells to undergo a form of programmed cell death. The discovery could be the basis for a new malaria vaccine [credit: Kurtis Lab/Big Ocean].
For the latest research, the team selected 12 resistant and 14 susceptible children from the Tanzanian cohort and looked at blood samples taken from the children around age two. Using a sophisticated method to introduce malaria proteins to each blood sample one by one, the researchers could look for any antibodies to a particular protein that were present in the resistant samples and not in the susceptible samples. That work identified PfGARP as a potential factor in conferring resistance.
Having identified PfGARP, the researchers then examined whether antibody responses to PfGARP were associated with resistance in a larger sample of 246 children. They found that children without anti-PfGARP antibodies were at 2.5 times higher risk of severe malaria compared to those who had the antibody.
The antibody
A series of laboratory experiments showed that the PfGARP protein is produced by malarial trophozoite cells, which live and feed off of nutrients inside red blood cells. The protein is then transported to the outer membrane of the red blood cell, where it makes the parasite cell vulnerable to the antibody.
“It’s a kill switch,” Kurtis said. “When the antibody binds to the protein, it sends a signal that tells the trophozoite to shrivel up and die. When we introduce the antibody to samples in petri dishes, we end up with 98 or 99 percent dead parasites.”
Having shown that PfGARP antibodies kill the parasite, the researchers developed two types of PfGARP vaccines. Both of those were shown to be protective in non-human primates exposed to a human form of malaria.
The researchers plan to continue testing different versions of the vaccine in animal models and ultimately to begin human trials in the coming years.
The study was published in the journal Nature.
Related topics
Antibodies, Drug Targets, Immunology, Vaccine
Related conditions
Malaria
Related organisations
Brown University, US National Institutes of Health (NIH), Warren Alpert Medical School of Brown University
Related people
Dr Jonathan Kurtis, Michal Fried, Patrick Duffy