Lysosomes key to coronavirus shedding, finds study
Posted: 29 October 2020 | Hannah Balfour (Drug Target Review) | No comments yet
Scientists reveal that coronaviruses de-activate lysosomes before using them to exit infected cells and spread through the body.
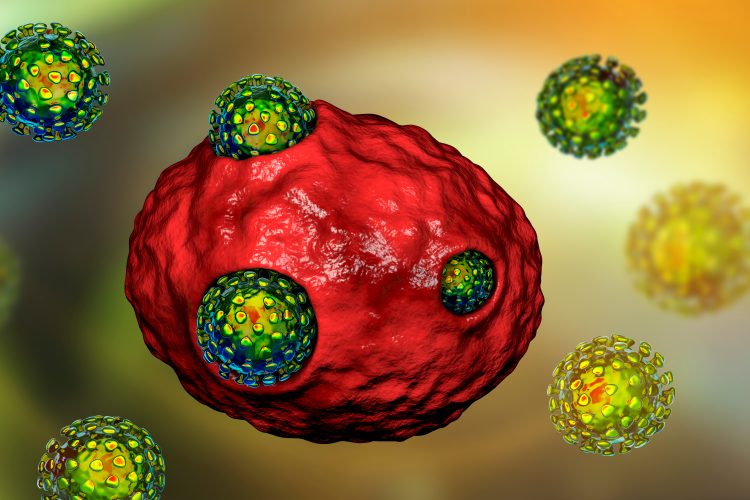

Researchers have discovered that SARS-CoV-2, the virus that causes COVID-19, uses lysosomes to exit cells. The team suggest that understanding this pathway, through which replicated viral particles are able to leave infected cells (shedding) and spread through the body, could be vital to stopping the transmission of SARS-CoV-2.
Using in vitro studies, scientists from the US National Institutes of Health (HIH) were able to demonstrate that SARS-CoV-2 deactivates the pathogen-destroying machinery of the lysosome, so instead of destroying the viruses, it allows SARS-CoV-2 particles to exit infected cells and spread through the body.
According to the team, targeting this lysosomal pathway could lead to the development of new, more effective antiviral therapies to fight COVID-19.
It is well established that viruses enter human host cells, hijack their intracellular protein assembly machinery and use it to replicate themselves. However, researchers have only a limited understanding of how viruses exit cells.
It has been thought that mot viruses (including influenza, hepatitis C and West Nile) exit through the biosynthetic secretory pathway, a pathway used by cells to transport hormones, growth factors and other materials to their surrounding environment. Researchers assumed that coronaviruses also use this pathway.

However, in a new experiment Dr Nihal Altan-Bonnet, chief of the Laboratory of Host-Pathogen Dynamics at the NIH’s National Heart, Lung and Blood Institute (NHLBI) and her post-doctoral fellow Dr Sourish Ghosh, found that when coronavirus-infected cells were exposed to chemical inhibitors of the biosynthetic pathway the virus was still able to exit the cells. Altan-Bonnet said: “This was the first clue that maybe coronaviruses were using another pathway.”
To identify the alternative pathway, the team designed additional experiments using microscopic imaging and virus-specific markers involving human cells. They discovered that coronaviruses somehow target the lysosomes, which are highly acidic, and congregate there.
But this raised a further question: If coronaviruses are accumulating in lysosomes and lysosomes are acidic, why are the coronaviruses not destroyed before exiting?
In a further series of experiments the researchers were able to demonstrate that the lysosomes of coronavirus-infected cells are de-acidified. According to the team, this weakens their destructive enzymes to the extent that the viruses remain intact and can go on to infect other cells when they exit.
The researchers also discovered that disrupting normal lysosome function appears to harm the cells’ immunological machinery. “We think this very fundamental cell biology finding could help explain some of the things people are seeing in the clinic regarding immune system abnormalities in COVID patients,” Altan-Bonnet said. This includes cytokine storms, in which an excess of certain pro-inflammatory proteins in the blood of COVID patients overwhelm the immune system and cause high death rates.
The team said that now they have identified the mechanism, they may be able to find a way to disrupt it and prevent lysosomes from delivering viruses to the outside of the cell; or re-acidify lysosomes to restore their normal functions. The authors have already identified one experimental enzyme inhibitor that potently inhibits viral shedding.
“The lysosome pathway offers a whole different way of thinking about targeted therapeutics,” Altan-Bonnet said, adding that further studies will be needed to determine if such interventions will be effective and whether existing drugs can help block this pathway. She notes the findings could go a long way toward stemming future pandemics caused by other coronaviruses that may emerge.
The findings were published in Cell.
Related topics
Analysis, Biomarkers, Cell-based assays, Disease Research, Drug Leads, Drug Targets, Imaging, In Vitro
Related conditions
Coronavirus, Covid-19, Hepatitis C, Influenza, West Nile virus
Related organisations
National Heart, National Heart Lung and Blood Institute (NHLBI), US National Institutes of Health (NIH)
Related people
Dr Nihal Altan-Bonnet, Dr Sourish Ghosh