Developing drugs for disordered proteins
Posted: 6 November 2020 | Hannah Balfour (Drug Target Review) | No comments yet
Scientists have developed a drug-like molecule to target amyloid-beta, a disordered protein implicated in Alzheimer’s disease that has been considered undruggable.
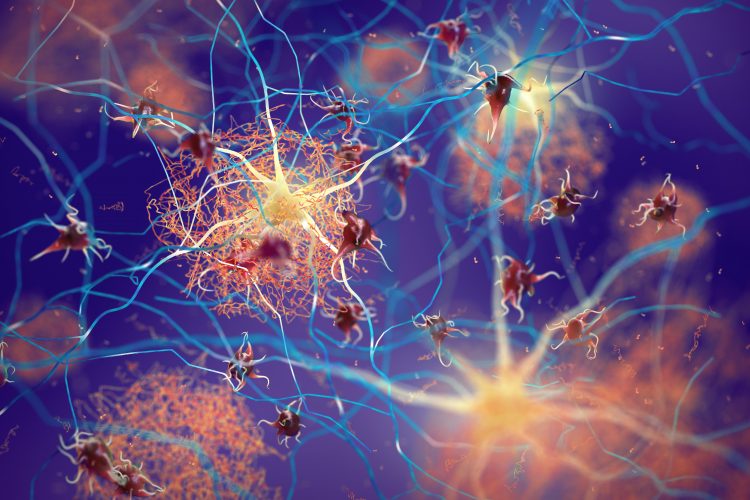

Three-dimensional (3D) illustration of amyloid beta plaques (brown mesh-like shapes) forming between neurons and disrupting their function. Amyloid plaques are a hallmark of Alzheimer’s Disease.
Researchers have developed small molecule drugs able to target disordered proteins, which have long been considered ‘undruggable’. In their study, the team designed a drug-like molecule to target amyloid beta (Aβ) monomers, the shape-shifting building blocks that go on to form aggregated plaques driving neurodegeneration in Alzheimer’s disease (AD).
Led by the University of Cambridge, UK, researchers from the UK’s Imperial College London, France’s Institut Pasteur and the University of Florence, Italy, found that it is possible for a drug-like molecule to target Aβ monomers in its disordered state, reducing their ability to form the toxic aggregates which are a hallmark of AD. According to the team, their research could form the basis of a new avenue for the development of potential treatments for the disease.
“Amyloid-beta is a disordered protein, a type of target that is elusive for standard therapeutic approaches,” said leader of the research, Professor Michele Vendruscolo from Cambridge’s Centre for Misfolding Diseases. “It is constantly changing shape, so traditional drug discovery techniques do not work on it. By revealing a new drug-binding mechanism, we have extended traditional drug discovery approaches based on the optimisation of the binding affinity to include disordered proteins.”
The researcher explained that traditional drug development relies on a lock-and-key mechanism; where, a stable protein acts as a lock and a specifically designed drug fits into the protein’s groves like a key. However, when a protein is disordered like Aβ, the shape of the lock changes and therefore it is difficult to develop drugs to bind to it. This is why they are considered undruggable.
In their study the team describes a new approach to getting drugs to bind, based on the ‘disordered binding mechanism’ that they discovered. Using this method, small molecules form a disordered complex with the protein target, the team explained that this looks as if the protein and the drug are ‘dancing’ with one another. The molecule they designed to form a disordered complex with Aβ is called 10074-G5.
The researchers characterised this new mechanism using a combination of biophysical experiments, mathematical modelling, in vivo experiments and computation.
First, they tested the aggregation of Aβ in the presence of 10074-G5 in in vitro assays. Data from these experiments allowed the researchers to build a mathematical model of how the drug was able to inhibit the aggregation of Aβ at the microscopic level.
The team also used high-performance computing methods to study the binding interaction at the atomic level. Further tests were then carried out in nematode worms, a model organism often used to study AD.
“In contrast to the traditional lock-and-key binding mechanism, in which a drug tightly interacts with its target in a specific conformation, we found that both the small molecule and the disordered protein remained extremely dynamic, and that the small molecule interacted with many parts of the protein,” said Gabriella Heller, Schmidt Science Fellow and the study’s first author.
“This way of stabilising native states of proteins is a powerful drug discovery strategy, which has so far been extremely challenging for disordered proteins,” said Vendruscolo.
The team said that despite their research being a long way off translation into clinic, it demonstrates that targeting the formation of senile plaques by preventing the aggregation of Aβ is a major therapeutic strategy for AD.
“Disordered proteins are also involved in a wide range of diseases including cancer and cardiovascular disease. We hope that we can extend this understanding to also target disordered proteins involved in other diseases,” concluded Heller.
The study was published in Science Advances.
Related topics
Drug Development, Drug Leads, Drug Targets, In Vitro, In Vivo, Neuroprotection, Neurosciences, Protein, Proteomics, Research & Development, Small Molecules, Therapeutics
Related conditions
Alzheimer's disease (AD), Cancer, cardiovascular disease (CVD)
Related organisations
Imperial College London, Institut Pasteur, University of Cambridge, University of Florence
Related people
Gabriella Heller, Professor Michele Vendruscolo