Novel inhibitor kills brain cancer cells missing ENO1 in mice, study shows
Posted: 24 November 2020 | Victoria Rees (Drug Target Review) | No comments yet
In pre-clinical studies, researchers have shown that a new therapy called POMHEX can destroy brain cancer cells that were missing one of two genes encoding the enolase enzyme.
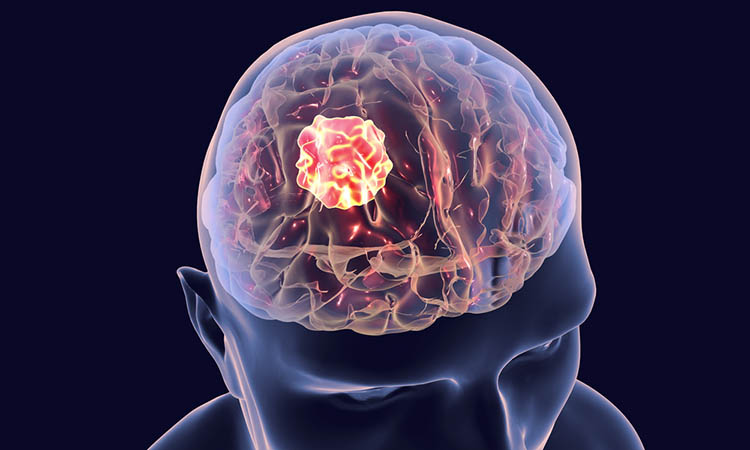

Researchers report that they have developed a novel targeted therapy, called POMHEX, which blocks critical metabolic pathways in cancer cells with specific genetic defects. According to the team, pre-clinical studies found the small molecule enolase inhibitor to be effective in killing brain cancer cells that were missing ENO1, one of two genes encoding the enolase enzyme.
The study was conducted at the University of Texas MD Anderson Cancer Center, US. The team say that enolase is an essential enzyme involved in glycolysis, a metabolic pathway that is elevated in many cancers to fuel their increased cell growth. Two genes, ENO1 and ENO2, encode slightly different but redundant versions of enolase and several cancers, such as glioblastoma, are missing the ENO1 gene because of chromosomal loss. This leaves the cancer cells with only ENO2 to continue glycolysis, making them highly sensitive to enolase inhibitors.
The researchers say the results provide proof-of-principle for a treatment strategy known as collateral lethality, in which an important protein is lost through genetic deletion as a bystander near a tumour suppressor gene and a redundant protein is blocked therapeutically.
“Collateral lethality could expand the scope of precision oncology beyond activated oncogenes and allow targeting of genomic deletions, largely considered un-actionable,” said corresponding author Assistant Professor Florian Muller. “Our work provides proof-of-principle that this approach can actually work with a drug in animal models.”
Therapies that target both forms of enolase have previously been developed, but blocking ENO1 can have unwanted side effects in normal cells. Targeting ENO2 specifically is attractive because it allows for the selective treatment of cancer cells missing ENO1.
The research team therefore worked to generate an enolase inhibitor, called HEX, that preferentially targets ENO2 over ENO1. To improve the drug’s ability to enter cells, the team created the prodrug POMHEX, which is biologically inactive until it is metabolised into HEX within cells.
In cancer cell lines lacking ENO1, the researchers found that treatment with POMHEX blocked glycolysis inhibited cell growth and stimulated cell death. Conversely, treatment of cells with normal ENO1 showed minimal effects.
Further, in animal models of ENO1-deficient tumours, both HEX and POMHEX treatment was well- tolerated and effectively blocked tumour growth relative to controls, with some instances of complete tumour eradication. Taking the work one step further, the team demonstrated that the therapeutically effective dose could be safely given in multiple models, suggesting favourable future translation to clinical studies.
“We were encouraged by the promising pre-clinical activity of these novel enolase inhibitors and that the safety profile extends to higher models. While there could be further refinements, I am optimistic that even HEX would show significant clinical activity against ENO1-deleted cancers,” Muller said.
The study was published in Nature Metabolism.
Related topics
Drug Discovery, Drug Targets, Oncology, Research & Development, Small Molecules, Target Molecule, Targets, Therapeutics
Related conditions
Brain cancer
Related organisations
University of Texas MD Anderson Cancer Center
Related people
Assistant Professor Florian Muller