Studies in mice reveal link between obesity and tumour growth
Posted: 10 December 2020 | Victoria Rees (Drug Target Review) | No comments yet
New research has provided a metabolic atlas for insights into obesity and tumours’ ability to hide from the immune system.


TIn a new study in mice, Harvard Medical School, US, researchers have detailed the link between cancer and obesity, with implications for cancer immunotherapy. The team found that obesity allows cancer cells to outcompete tumour-killing immune cells in a battle for fuel.
The research team showed that a high-fat diet reduces the numbers and antitumour activity of CD8+ T cells inside tumours.
“Putting the same tumour in obese and non-obese settings reveals that cancer cells rewire their metabolism in response to a high fat diet,” said Professor Marcia Haigis, co-senior author of the study. “This finding suggests that a therapy that would potentially work in one setting might not be as effective in another, which needs to be better understood given the obesity epidemic in our society.”
The researchers found that tumours grew much more rapidly in animals on high-fat diets compared to those on normal diets. However, this occurred only in cancer types that are immunogenic, which can contain high numbers of immune cells, are more easily recognised by the immune system and are more likely to provoke an immune response.
Experiments revealed that diet-related differences in tumour growth depended specifically on the activity of CD8+ T cells. Diet did not affect tumour growth rate if CD8+ T cells were eliminated experimentally in mice.
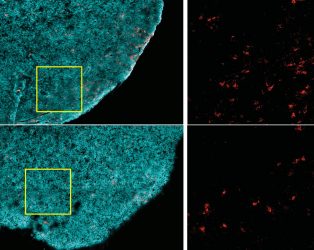

Tumours from a non-obese animal (top row) contain more CD8+ T cells (red), compared to those from an obese one (bottom row). Tumour cells are highlighted in cyan [credit: Ringel et al, 2020].
The researchers found that high-fat diets reduced the presence of CD8+ T cells in the tumour microenvironment, but not elsewhere in the body. Those remaining in the tumour were less robust – they divided more slowly and had markers of decreased activity. When these cells were isolated and grown in a lab, they had normal activity, suggesting something in the tumour impaired these cells’ function.
The team also encountered an apparent paradox. In obese animals, the tumour microenvironment was depleted of key free fatty acids, a major cellular fuel source, even though the rest of the body was enriched in fats, as expected in obesity.
This pushed the researchers to craft a comprehensive atlas of the metabolic profiles of different cell types in tumours under normal and high-fat diet conditions.
The analyses revealed that cancer cells adapted in response to changes in fat availability. Under a high-fat diet, cancer cells were able to reprogramme their metabolism to increase fat uptake and utilisation, while CD8+ T cells did not. This ultimately depleted the tumour microenvironment of certain fatty acids, leaving T cells starved for this fuel.
The researchers investigated the effects of obesity on mouse models of different types of cancer, including colorectal, breast, melanoma and lung. Led by study co-first authors Alison Ringel and Jefte Drijvers, the team gave mice normal or high-fat diets, the latter leading to increased body weight and other obesity-related changes.
Through several different approaches, including single-cell gene expression analyses, large-scale protein surveys and high-resolution imaging, the team identified numerous diet-related changes to metabolic pathways of both cancer and immune cells in the tumour microenvironment.
Of interest was PHD3, a protein that in normal cells has been shown to act as a brake on excessive fat metabolism. Cancer cells in an obese environment had significantly lower expression of PHD3 compared to in a normal environment. When the researchers forced tumour cells to overexpress PHD, they found that this diminished a tumour’s ability to take up fat in obese mice. It also restored the availability of key free fatty acids in the tumour microenvironment.
Increased PHD3 expression largely reversed the negative effects of a high-fat diet on immune cell function in tumours. Those with high PHD3 grew slower in obese mice compared to tumours with low PHD3. This was a direct result of increased CD8+ T-cell activity. In obese mice lacking CD8+ T cells, tumour growth was unaffected by differences in PHD3 expression.
“CD8+ T cells are the central focus of many promising precision cancer therapies, including vaccines and cell therapies such as CAR T,” said co-senior author Professor Arlene Sharpe. “These approaches need T cells to have sufficient energy to kill cancer cells, but at the same time we do not want tumours to have fuel to grow. We now have amazingly comprehensive data for studying this dynamic and determining mechanisms that prevent T cells from functioning as they should.”
“Our study provides a high-resolution metabolic atlas to mine for insights into obesity, tumour immunity and the crosstalk and competition between immune and tumour cells. There are likely many other cell types involved and many more pathways to be explored,” Haigis said.
The study was published in Cell.
Related topics
Disease Research, Drug Targets, Immuno-oncology, Immuno-oncology therapeutics, Immunotherapy, T cells, Targets
Related organisations
Harvard Medical School
Related people
Alison Ringel, Jefte Drijvers, Professor Arlene Sharpe, Professor Marcia Haigis