Novel intestine model could accelerate the development of treatments
Posted: 8 January 2021 | Hannah Balfour (Drug Target Review) | No comments yet
The transparent millifluidic perfusion cassettes (mPCs) developed by researchers should allow for better evaluations of the interactions between the cells and invading pathogens.
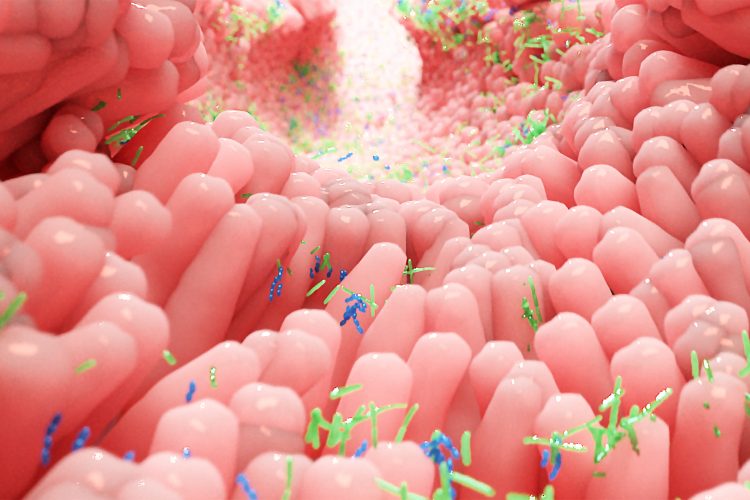

Scientists have developed a tool to simulate the human intestine, giving them a mechanical model for the real-time growth of bacterial infections that should make it more practical to find treatments for diseases like infectious diarrhoea.
![Figure 1: Researchers at Rice University and Baylor College of Medicine developed millifluidic perfusion cassettes (mPCs) that mimic conditions in the intestines to evaluate infections like those that cause diarrhea. The devices formed from 3D-printed molds (top right) were seeded with intestinal enteroid cultures (IECs) and infected with pathogens for 24 hours or more to see how infections take hold [Credit: Rice University/Baylor College of Medicine].](https://www.drugtargetreview.com/wp-content/uploads/millifluidic-perfusion-cassettes-mPCs-intestines.jpg)
![Figure 1: Researchers at Rice University and Baylor College of Medicine developed millifluidic perfusion cassettes (mPCs) that mimic conditions in the intestines to evaluate infections like those that cause diarrhea. The devices formed from 3D-printed molds (top right) were seeded with intestinal enteroid cultures (IECs) and infected with pathogens for 24 hours or more to see how infections take hold [Credit: Rice University/Baylor College of Medicine].](https://www.drugtargetreview.com/wp-content/uploads/millifluidic-perfusion-cassettes-mPCs-intestines.jpg)
Figure 1: Researchers at Rice University and Baylor College of Medicine developed millifluidic perfusion cassettes (mPCs) that mimic conditions in the intestines to evaluate infections like those that cause diarrhea. The devices formed from 3D-printed molds (top right) were seeded with intestinal enteroid cultures (IECs) and infected with pathogens for 24 hours or more to see how infections take hold [Credit: Rice University/Baylor College of Medicine].
The collaborative team, led by bioengineer Jane Grande-Allen of Rice University’s Brown School of Engineering, US, developed transparent millifluidic perfusion cassettes (mPCs) that are easy to fabricate and operate – and are also compatible with common microscopic and biochemical analysis.
The mPCs are seeded with human intestinal enteroids (HIEs), cultures that contain the major cell types native to the intestinal epithelium, and are about the size of standard 96-well plates. When fluid containing bacteria is flowed through, it forms a biofilm on the cells, a phenomenon which the team said cannot be seen in static platforms. They also have micro-scale ports for input and output, allowing for sampling of the environment.
Reduce preclinical failures with smarter off-target profiling
24 September 2025 | 15:00PM BST | FREE Webinar
Join this webinar to hear from Dr Emilie Desfosses as she shares insights into how in vitro and in silico methods can support more informed, human-relevant safety decisions -especially as ethical and regulatory changes continue to reshape preclinical research.
What you’ll learn:
- Approaches for prioritizing follow-up studies and refining risk mitigation strategies
- How to interpret hit profiles from binding and functional assays
- Strategies for identifying organ systems at risk based on target activity modulation
- How to use visualization tools to assess safety margins and compare compound profiles
Register Now – It’s Free!
According to the scientists, researchers can easily evaluate how well bacteria adhere to and infect the cells, both visually and by sampling through the ports on either end. Grande-Allen added that a benefit of their mPCs over static plates, is that the slow flow allows extended observation and more realistic results.
In a study published in Annals of Biomedical Engineering, the researchers tested the device and found the first direct evidence that aggregative adherence fimbriae, sticky appendages found on most infectious enteroaggregative Escherichia coli (EAEC), are necessary for the bacteria to form a biofilm.
“Our intention was to slowly flush the bacterial toxins to keep them from harming the cells,” Grande-Allen said. “That allowed more cells to survive so we could watch the process of infection. We were completely surprised to see that it also dramatically changed the nature of the film that formed.”
She added that the flow allows for oxygen and nutrients to be replenished in the chamber, prolonging the life of the HIE cultures and allowing for better evaluation of realistic interactions between the cells and invading pathogens.
Grande-Allen concluded that mPCs will facilitate research on many such interactions, commenting: “This will allow the systematic examination of a lot of different combinations, good and bad, and how flow changes the dynamics of this environment.”
Related topics
Analytical Techniques, Biofilms, Cell Cultures, Cell-based assays, Disease Research, Drug Discovery, In Vitro, Microfluidic Technology
Related conditions
Diarrhea
Related organisations
Rice University
Related people
Jane Grande-Allen