Researchers link cellular transport pathway to glioblastoma
Posted: 18 January 2021 | Victoria Rees (Drug Target Review) | No comments yet
A study has said that restoring a particular protein’s activity could be a new therapeutic strategy to treat glioblastoma.

Researchers have identified a new cellular pathway that limits the growth and spread of brain tumours by controlling the recycling of cell surface receptor proteins. The team suggest that the pathway, which involves a protein called Rab35, is defective in many patients with glioblastoma and that restoring Rab35’s activity could be a new therapeutic strategy for this deadly form of brain cancer. The study was conducted at McGill University, Canada.
According to the researchers, in normal, healthy cells, transport pathways are regulated by a class of protein known as small GTPases. “We previously demonstrated that the levels of a small GTPase called Rab35 are decreased in human glioblastomas,” said Peter McPherson, one of the lead researchers. “We thus sought to investigate the role of Rab35 in the progression of glioblastoma.”
The researchers found that reducing the levels of Rab35 increased the growth and spread of brain tumours in mice, thereby shortening the animals’ life span. In contrast, elevating Rab35 levels reduced tumour growth and prolonged the animals’ survival.
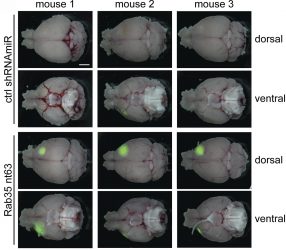

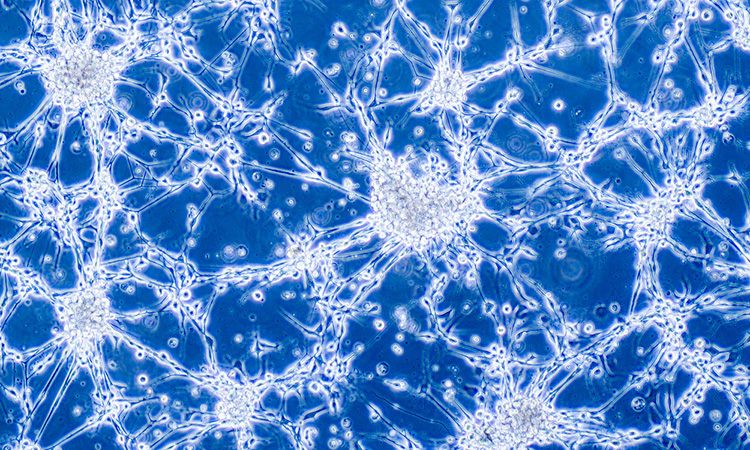
Compared with control mice (top two rows), brain tumours (green) grow faster in the absence of Rab35 (bottom two rows) [credit: Kulasekaran et al. Originally published in Journal of Cell Biology. doi.org/10.1083/jcb.202004229].
They discovered that Rab35 is activated by a pathway involving another small GTPase called Arf5. Together, Arf5 and Rab35 appear to control the transport of various cell surface receptors, thereby restricting the ability of cells to migrate and invade through tissues, limiting the ability of brain tumour-initiating cells to replicate.
One of the cell surface receptors controlled by this pathway is the signalling protein epidermal growth factor receptor (EGFR). In glioblastoma cells lacking Rab35, EGFR is increasingly recycled to the cell surface instead of being degraded, enhancing the receptor’s signalling activity. The researchers found that inhibiting EGFR with the cancer drug erlotinib reduced the production of a protein called SPOCD1, which is known to promote the proliferation and/or metastasis of multiple cancers.
The team suggest that restoring the activity of Rab35 might limit the development of glioblastomas by altering the degradation and recycling of multiple cell surface receptors, including EGFR.
“Rab GTPases are emerging as an important new set of drug targets in cancer,” McPherson said. “Our study reveals an unprecedented link between Rab and Arf proteins and identifies new loci for therapeutic intervention in glioblastoma.”
The results were published in Journal of Cell Biology.
Related topics
Drug Targets, Oncology, Protein, Proteomics, Research & Development, Targets
Related conditions
Glioblastoma
Related organisations
McGill University
Related people
Peter McPherson