Could synNotch CAR T cells be the answer to fighting solid tumours?
Posted: 22 January 2021 | Hannah Balfour (Drug Target Review) | No comments yet
Researchers have developed a novel CAR T-cell therapy for neuroblastoma which uses gating to limit toxicity and T-cell exhaustion.

A novel chimeric antigen receptor T-cell (CAR T) therapy targeting neuroblastoma effectively killed cancer cells while sparing healthy tissues in pre-clinical studies. While this work is in the preclinical phase, the developers of the treatment said it opens the door for other potentially life-saving treatments for patients with solid tumours.
While CAR-T therapies have revolutionised the treatment of haematological malignancies, like leukaemia, they have so far shown little efficacy in solid tumours, including childhood cancers such as neuroblastoma.
CAR T therapies consist of a patient’s own immune system T cells, engineered to recognise a specific antigen expressed by a cancerous tissue and destroy those cells. According to Dr Shahab Asgharzadeh, a physician scientist at the Cancer and Blood Disease Institute of Children’s Hospital Los Angeles (CHLA), who worked on the new therapy, CAR T therapy works in leukaemia, despite the antigen that the T cells target being expressed on healthy blood cells, because the toxic effects of these B cells being destroyed can be safely treated.
However, in solid tumours, like neuroblastoma, many of the antigens expressed by the tumour are also found in healthy tissues, where the toxicity cannot be safely managed. Because of this and the immunosuppressive microenvironment of the solid tumour, preclinical studies with CAR-T therapies targeting these cancers have resulted in little efficacy or unacceptable levels of toxicity.
“CAR-T therapy is incredibly powerful, but for solid tumours it has significant barriers,” said Dr Babak Moghimi, the first author of the study published in Nature Communications. “We needed a way to boost the CAR T-cells to make them fight harder and smarter against the cancer. But we also want to save brain cells and other healthy tissue.”
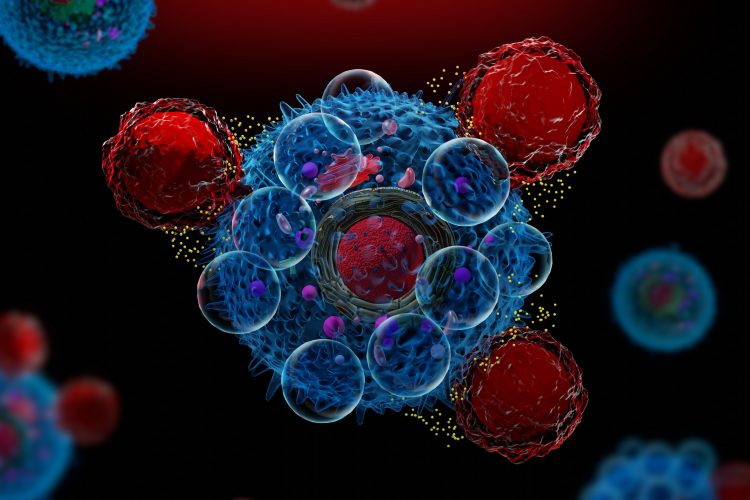
To minimise toxicity, they used a new CAR-T technology called synthetic Notch (synNotch). SynNotch CAR T-cells have a unique property, called gating, that allows them to target specific cancers very precisely. The gating function works similarly to logic gates, a tool often used by computer programmers: if condition A is met, then do action B.
Dr Moghimi explained that the special synNotch protein on the surface of the T cell is designed to recognise the antigen GD2. When it does, the synNotch protein instructs the T cell to activate its CAR T properties, enabling its ability to recognise a second antigen, B7H3. Because the T cell has to follow these specific instructions, it can only kill cells with both antigens. While healthy cells may express low levels of one of the antigens, they should not express both, where neuroblastoma cells do.
The team was also able to overcome T-cell exhaustion. “With normal CAR-T therapy,” said Dr Asgharzadeh, “the CAR T-cells burn out and are no longer active after some time. But we discovered the synNotch CAR T-cells are more metabolically stable because they are not activated constantly.” As a result, they use less energy and can continue to fight the cancer for a longer period of time.
Related topics
Chimeric Antigen Receptors (CARs), Drug Development, Drug Targets, Immuno-oncology, Immunotherapy, In Vivo, T cells
Related conditions
Leukaemia, Neuroblastoma
Related organisations
Children's Hospital Los Angeles (CHLA)
Related people
Dr Shahab Asgharzadeh