Tissue damage and genetic mutations interact to drive pancreatic cancer, finds study
Posted: 4 February 2021 | Hannah Balfour (Drug Target Review) | No comments yet
New research shows tissue damage to cells carrying KRAS mutations induces epigenetic changes that promote pancreatic cancer.
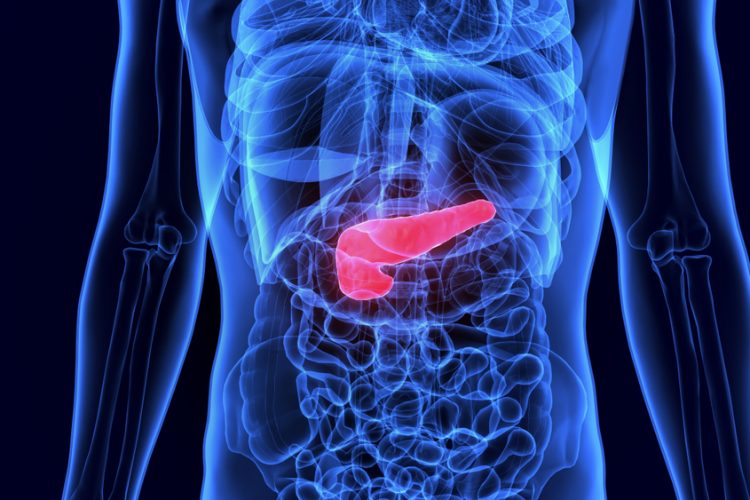

According to researchers, tissue damage synergises with specific genetic changes to promote the onset of pancreatic cancer. In addition, the team found that over half of the epigenetic changes that typically characterise advanced pancreatic cancer were already present in these cells within 48 hours of tissue damage.
The team explained that pancreatic cancer is not alone in forming from the site of tissue damage, as many other cancers form the same way, for example, stomach cancer caused by Helicobacter pylori infection, Barrett’s oesophagus caused by acid reflux, as well as smoking-induced lung cancer.
However, exactly how tissue damage interacts with genetic changes to promote cancer is not fully understood, because most knowledge concerns cancers in their advanced stages, not the early ones. This is true of pancreatic cancer, which is typically diagnosed very late.
To combat this, researchers in Scott Lowe’s lab at the Sloan Kettering Institute, US, are trying to study the earliest stages of pancreatic cancer development.
“If we understood how these tumours form, maybe we could catch them before the cancer has progressed to an incurable stage,” commented Dr Direna Alonso Curbelo, a postdoctoral fellow in the Lowe lab and the first author of the new paper published in Nature.
In the study, they used advanced genomic techniques and mouse models to discern how the combination of tissue damage and the KRAS gene mutation synergise to promote the earliest stages of pancreatic cancer.
According to the team, the process begins when the pancreas attempts to repair damage caused by its own digestive enzymes. Dr Alonso Curbelo explained that the misplaced release of these enzymes can degrade pancreatic tissue and cause pancreatitis, a form of inflammation.
To repair the damage, the cells in the affect area change their behaviour, temporarily shut down their production of digestive enzymes while repairs take place, before returning to their normal functions once the damage has been resolved.
However, the researchers observed that in cells containing the KRAS mutation, once the wound-healing response is activated the cells never return to their normal function. KRAS mutations are known to drive tumor growth in 95 percent of people with pancreatic cancer.
But how does mutant KRAS derail the wound-healing process to initiate the disease? According to the new study, damage to KRAS-mutant cells induces major epigenetic changes. These differences in the organisation of the chromatin alter the expression of various genes, resulting in a malignant phenotype.
The researchers added that this restructuring process occurs very quickly, with over half of the chromatin changes that typically characterise advanced pancreatic cancer appearing within 48 hours of tissue damage.
Importantly, said the researchers, their discovery could enable scientists to prevent cancer development by interfering with the activation of genes that become inappropriately turned on. Something which the researchers demonstrated in mice in their study.
“Our study provides some understanding of why cancers often arise at sites of tissue damage,” Dr Lowe commented. “You need both a mutated gene and tissue damage to activate the epigenetic program that drives cancer initiation. Each alone does not do it.”
The team concluded that the specificity of the epigenetic changes driving pancreatic cancer initiation, opens the possibility that scientists could try to target these newly turned-on genes as a way to selectively interrupt cancer development. In addition, these genes could be used as biomarkers to help identify cancer earlier and enable interventions before it progresses too far.
Related topics
Disease Research, Drug Targets, Epigenetics, Genomics, Oncology
Related conditions
Lung cancer, Pancreatic cancer, Stomach cancer
Related organisations
Sloan Kettering Institute
Related people
Dr Direna Alonso Curbelo, Scott Lowe