Vaccine strategy developed to fight urinary tract infections
Posted: 2 March 2021 | Victoria Rees (Drug Target Review) | No comments yet
Researchers have shown that administering a urinary tract infection vaccine directly to the bladders of mice helped them to effectively fight bacteria.


Researchers have described a new vaccination strategy that could re-programme the body to combat the bacteria that cause urinary tract infections (UTIs). The study was conducted at Duke University, US.
“Although several vaccines against UTIs have been investigated in clinical trials, they have so far had limited success,” said Professor Soman Abraham, senior author on the paper. “There are currently no effective UTI vaccines available for use in the US in spite of the high prevalence of bladder infections. Our study describes the potential for a highly effective bladder vaccine that can not only eradicate residual bladder bacteria, but also prevent future infections.”
The strategy, which the team showed to be effective in mouse models, involves re-programming an inadequate immune response that the team identified last year. They observed that when mouse bladders get infected with E. coli bacteria, the immune system dispatches repair cells to heal the damaged tissue, while launching very few warrior cells to fight off the attacker. This causes bacteria to never fully clear, living on in the bladder to attack again.
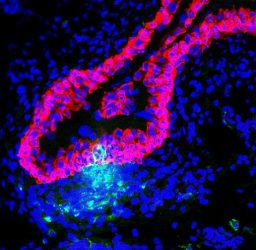

Immune cells (green) infiltrating the bladder lining (red) to fight off invading bacteria. Blue areas are nuclei of other bladder tissue cells [credit: Duke Health].
“The new vaccine strategy attempts to ‘teach’ the bladder to more effectively fight off the attacking bacteria. By administering the vaccine directly into the bladder where the residual bacteria harbour, the highly effective vaccine antigen, in combination with an adjuvant known to boost the recruitment of bacterial clearing cells, performed better than traditional intramuscular vaccination,” said lead author of the study, Dr Jianxuan Wu.
The researchers reported that bladder-immunised mice effectively fought off infecting E. coli and eliminated all residual bladder bacteria, suggesting the site of administration could be an important consideration in determining the effectiveness of a vaccine.
“We are encouraged by these findings and since the individual components of the vaccine have previously been shown to be safe for human use, undertaking clinical studies to validate these findings could be done relatively quickly,” Abraham said.
The results of the study were published in PNAS.
Related topics
Immunology, In Vivo, Vaccine
Related conditions
Urinary tract infection (UTI)
Related organisations
Duke University
Related people
Dr Jianxuan Wu, Professor Soman Abraham