Unpredicted binding found between SARS-CoV-2 and hepatitis C drug, telaprevir
Posted: 24 March 2021 | Victoria Rees (Drug Target Review) | No comments yet
Insights into the interaction between telaprevir and the SARS-CoV-2 main protease could aid in future COVID-19 drug design, say scientists.

Scientists have found new, unexpected behaviours when SARS-CoV-2 – the virus that causes COVID-19 – encounters drug inhibitors, which bind to certain components of the virus and block its ability to reproduce.
According to the team, from the US Department of Energy’s Oak Ridge National Laboratory, the research provides key insights for advancing drug design and drug repurposing efforts to treat COVID-19.
The researchers used neutron scattering to investigate interactions between telaprevir, a drug used to treat hepatitis C viral infection and the SARS-CoV-2 main protease (Mpro), the enzyme responsible for enabling the virus to reproduce.
They discovered unforeseen changes in the electric charges in the drug binding site of the protease enzyme that were not predicted by prevailing computer simulations.
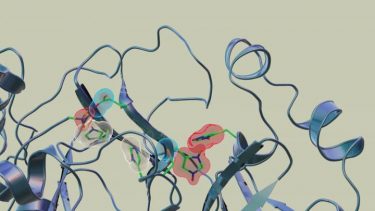

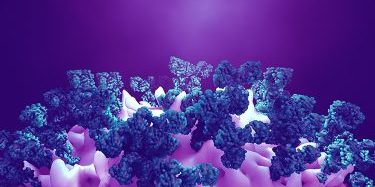
Neutron scattering experiments show that when telaprevir binds to the protease, the amino acid sites will change their electric charges by altering their protonation states [credit: Jill Hemman and Michelle Lehman/ORNL, US Department of Energy].
“We found this particular protein – the SARS-CoV-2 Mpro – which a lot of researchers are studying by computational methods, is behaving in a surprising way,” said lead author Daniel Kneller. “Our findings provide critical experimental data needed to improve computer modelling so that simulations more closely match reality.”
The virus reproduces by assembling polyproteins made from almost 2,000 amino acids that must be cut into smaller chains by the Mpro. Finding a drug that effectively blocks or inhibits the protease function is paramount to preventing the virus from replicating and spreading to other healthy cells in the body.
In previous studies, the team used neutron and X-ray scattering to build a complete map of every atom in the SARS-CoV-2 protease enzyme. They located the sites where a drug inhibitor would bind to the protease enzyme and mapped the network of hydrogen bonds that hold the protease together. They also determined the locations of the positive, negative and neutral electric charges in the protease amino acid sites where the polyprotein cutting action occurs.
“In this study, we discovered that when telaprevir bound to the Mpro, the protonation states [proton addition or loss] in the binding site altered, meaning the locations of the hydrogen atoms changed; essentially, some amino acid sites either gained or lost hydrogen atoms, which changed their electric charges,” said corresponding author Andrey Kovalevsky. “However, the overall electric charge of the protease inhibitor binding site remained the same as it was before it was attached to the drug molecule. That is something we did not expect and was not correctly predicted in computer simulations.”
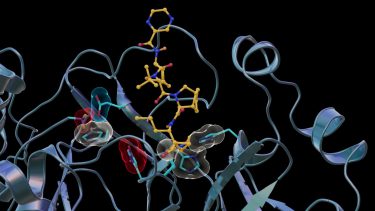

Neutron scattering experiments show electric charges, shown in red, blue and grey, in the SARS-CoV-2 Mpro site where telaprevir binds to the structure. The experiments provide critical data for the design of small-molecule drugs to treat COVID-19 [credit: Jill Hemman and Michelle Lehman/ORNL, US Department of Energy].
The researchers said their observations suggest the protease enzyme can alter its protonation states when it binds to certain drugs and therefore assumptions about binding behaviours should not be based purely on the properties of the protease structure before an inhibitor is bound.
“Protein behaviour at the level of individual atoms is notoriously difficult to predict. Simulations have to be designed based off ideal scenarios of general chemical knowledge and mathematics, but proteins do not always adhere to ideal scenarios,” Kneller said.
Telaprevir is one of a number of US Food and Drug Administration (FDA)-approved drug inhibitors used to treat hepatitis C viral infection. It belongs to a class of agents called covalent peptidomimetic inhibitors that work by using strings of unnatural amino acids to bind to specific target proteins.
“We have shown that some of the hepatitis C virus protease inhibitors can also inhibit the SARS-CoV-2 Mpro,” said Leighton Coates, the paper’s co-corresponding author. “In this study, we chose telaprevir because we were able to produce high-quality crystal samples that we could study with neutrons. This should have a direct impact on drug design. We believe what we learn from the interactions between telaprevir and the protease enzyme should be transferable to other covalent peptidomimetic inhibitors such as those being investigated right now by major pharmaceutical companies.”
The study was published in the Journal of Medicinal Chemistry.
Related topics
Drug Development, Imaging, Informatics, Molecular Targets, Structural Biology
Related conditions
Covid-19
Related organisations
Oak Ridge National Laboratory (ORNL), US Food and Drug Administration (FDA)
Related people
Andrey Kovalevsky, Daniel Kneller, Leighton Coates