Researchers discover strategy to reverse poor stress response in mice
Posted: 8 April 2021 | Victoria Rees (Drug Target Review) | No comments yet
Studying the mouse gene Ophn1, researchers have found its removal causes stressed behaviours, also identifying three ways to reverse this effect.

According to scientists, in humans, mutations in the OPHN1 gene cause a rare X-linked disease that includes poor stress tolerance. Now, researchers at the Cold Spring Harbor Laboratory (CSHL), US, have studied the mouse gene Ophn1, an analogue of the human gene, which plays a critical role in developing brain cell connections, memories and stress tolerance. They found that when Ophn1 was removed in a specific part of the brain, mice expressed depression-like helpless behaviours. The researchers discovered three ways to reverse this effect.
To test for stress, the researchers put mice into a two-room cage with a door in between. Normal mice escape from the room that gives them a light shock on their feet. However animals lacking Ophn1 sit helplessly in that room without trying to leave.
The researchers developed a way to delete the Ophn1 gene in different brain regions. They found that removing Ophn1 from the prelimbic region of the medial prefrontal cortex (mPFC), an area known to influence behavioural responses and emotion, induced the helpless phenotype. Then the team figured out which brain circuit was disrupted by deleting Ophn1, creating overactivity in the brain region and ultimately the helpless phenotype.
According to the team, pyramidal neurons are central to this brain circuit. If they fire too much, the mouse becomes helpless. Another cell, an interneuron, regulates the pyramidal neuron activity, making sure it does not fire too much. These two cells feedback to each other, creating a loop.
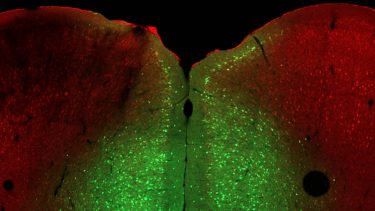

In this image of a mouse brain, the green colour shows the prelimbic region of the medial prefrontal cortex, where the researchers injected a virus to delete Ophn1. Ophn1 (red) is still present in other parts of the brain. The researchers discovered that deleting the gene in only this part of the brain caused the observed failure in stress adaptation. Human brains are organised similarly, so their findings in mice may be applicable to helping human patients who experience an inability to deal with stressful situations [credit: Minghui Wang/Van Aelst Lab, CSHL/2021].
The team say that Ophn1 controls a particular protein, RhoA kinase, within this feedback loop which helps regulate and balances activity.
The researchers found three agents that reversed the helpless phenotype. Fasudil, an inhibitor specific for RhoA kinase, mimicked the effect of the missing Ophn1. A second drug dampened excess pyramidal neuron activity. A third drug woke up the interneurons to inhibit pyramidal neurons.
“So bottom line, if you can restore the proper activity in the medial prefrontal cortex, then you could rescue the phenotype. So that was actually very exciting,” said lead researcher Professor Linda Van Aelst.
Van Aelst hopes that understanding the complex feedback loop behind Ophn1-related stress responses will lead to better treatments for stress in humans.
The findings from the study are published in Neuron.
Related topics
Drug Discovery, In Vivo, Molecular Targets, Target Validation, Therapeutics
Related conditions
Stress
Related organisations
Cold Spring Harbor Laboratory (CSHL)
Related people
Professor Linda Van Aelst