Novel PF74-like small molecules developed to target HIV capsid protein
Posted: 9 April 2021 | Victoria Rees (Drug Target Review) | No comments yet
Scientists have developed PF74-like small molecules able to target the HIV-1 capsid protein, identifying four potent compounds.

Novel PF74-like small molecules that target the HIV-1 capsid protein (CA) have been discovered by a team from the University of Minnesota in collaboration with Emory University, both US.
“The capsid core provides a protected environment for viral reverse transcription and shields viral genome products from host nucleic acid sensing. CA-CA interactions drive capsid assembly and impact capsid disassembly and core stability. Perturbation of core stability results in premature uncoating, impaired reverse transcription, and attenuated infection,” the authors of the study write in their paper.
According to the researchers, of all known small molecules targeting the HIV CA, PF74 represents by far the best characterised chemotype, due to its ability to confer antiviral phenotypes in both early and late phases of viral replication. However, the prohibitively low metabolic stability renders PF74 a poor antiviral lead.
The authors of the study report on their medicinal chemistry efforts towards identifying novel and metabolically stable small molecules targeting the PF74 binding site. Specifically, they replaced the inter-domain-interacting, electron-rich indole ring of PF74 with less electron-rich isosteres, including imidazolidine-2,4-dione, pyrimidine-2,4-dione and benzamide.
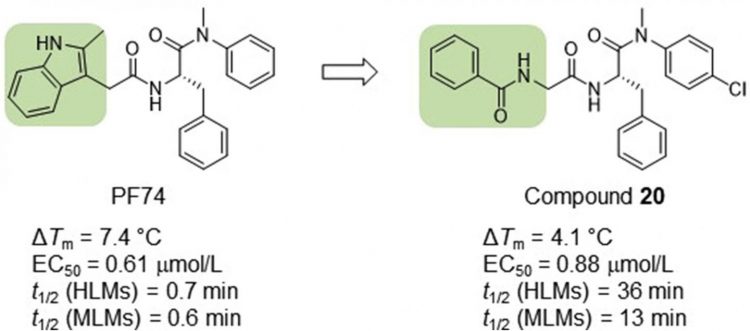

HIV-1 capsid protein (CA)-targeting PF74 suffers from prohibitively low metabolic stability. Structural modifications have yet to produce analogues with balanced potency and metabolic stability. Replacing the inter-domain interacting indole ring with less electron-rich isosteres allowed the identification of compound 20 with drastically improved metabolic stability while largely maintaining antiviral potency [credit: Acta Pharmaceutica Sinica B].
They identified four potent antiviral compounds (10, 19, 20 and 26) with markedly improved metabolic stability. Compared to PF74, the team found that analogue 20 exhibited similar submicromolar potency and much longer (51-fold) half-life in human liver microsomes (HLMs). Conducting molecular docking studies, the researchers confirmed that 20 binds to the PF74 binding site and revealed distinct binding interactions conferred by the benzamide moiety.
The research team conclude that their data supports compound 20 as a promising antiviral lead against HIV.
The study was published in Acta Pharmaceutica Sinica B.
Related topics
Drug Development, Drug Discovery, Medicinal Chemistry, Small Molecules, Therapeutics
Related conditions
HIV
Related organisations
Emory University, University of Minnesota