Experimental drug shown to reverse effects of Alzheimer’s in mice
Posted: 23 April 2021 | Victoria Rees (Drug Target Review) | No comments yet
Oral doses of a new drug named CA administered to mouse models led to improvements in Alzheimer’s disease, including memory.

Researchers at the Albert Einstein College of Medicine, US, have designed an experimental drug that reversed key symptoms of Alzheimer’s disease in mice.
“We were encouraged to find in our study that the drop-off in cellular cleaning that contributes to Alzheimer’s in mice also occurs in people with the disease, suggesting that our drug may also work in humans,” said co-study leader Dr Ana Maria Cuervo.
In the 1990s, Cuervo discovered the existence of this cell-cleaning process, known as chaperone-mediated autophagy (CMA). According to Cuervo, CMA becomes less efficient as people age, increasing the risk that unwanted proteins will accumulate into insoluble clumps that damage cells.
Automation now plays a central role in discovery. From self-driving laboratories to real-time bioprocessing
This report explores how data-driven systems improve reproducibility, speed decisions and make scale achievable across research and development.
Inside the report:
- Advance discovery through miniaturised, high-throughput and animal-free systems
- Integrate AI, robotics and analytics to speed decision-making
- Streamline cell therapy and bioprocess QC for scale and compliance
- And more!
This report unlocks perspectives that show how automation is changing the scale and quality of discovery. The result is faster insight, stronger data and better science – access your free copy today
The team first looked at whether impaired CMA contributes to Alzheimer’s. To do so, they genetically engineered a mouse to have excitatory brain neurons that lacked CMA. The absence of CMA in one type of brain cell was enough to cause short-term memory loss, impaired walking and other problems often found in rodent models of Alzheimer’s disease. In addition, the absence of CMA profoundly disrupted proteostasis – the cells’ ability to regulate the proteins they contain. Normally soluble proteins had shifted to being insoluble and at risk for clumping into toxic aggregates.
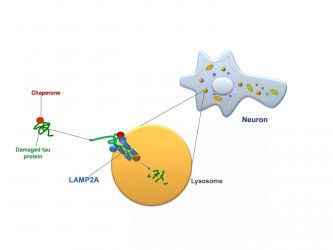

Chaperone-mediated autophagy in a neuron: A protein chaperone (red circle) escorting a molecule of damaged tau protein (green) docks with a LAMP2 receptor (blue) on the cell’s lysosome (orange) and pushes the tau protein inside the lysosome, where it is digested [credit: Albert Einstein College of Medicine].
The team studied a mouse model of early Alzheimer’s in which brain neurons were made to produce defective copies of the protein tau. They focused on CMA activity within neurons of the hippocampus – the brain region crucial for memory and learning and found that CMA activity in those neurons was significantly reduced compared to control animals.
The researchers then looked at single-cell RNA-sequencing data from neurons obtained post-mortem from the brains of Alzheimer’s patients and from a comparison group of healthy individuals. The sequencing data revealed CMA’s activity level in patients’ brain tissue. They found that CMA activity was somewhat inhibited in people who had been in the early stages of Alzheimer’s, followed by much greater CMA inhibition in the brains of people with advanced Alzheimer’s.
“By the time people reach the age of 70 or 80, CMA activity has usually decreased by about 30 percent compared to when they were younger,” said Cuervo. “If you add neurodegenerative disease to the mix, the effect on the normal protein makeup of brain neurons can be devastating. Our study shows that CMA deficiency interacts synergistically with Alzheimer’s pathology to greatly accelerate disease progression.”
In CMA, proteins called chaperones bind to damaged or defective proteins in cells of the body. The chaperones ferry their cargo to the cells’ lysosomes, which digest and recycle waste material. To successfully get their cargo into lysosomes, however, chaperones must first “dock” the material onto a protein receptor called LAMP2A that sprouts from the membranes of lysosomes. The more LAMP2A receptors on lysosomes, the greater the level of CMA activity possible.
The team developed a novel drug that shows potential for treating Alzheimer’s. The new drug, called CA, works by increasing the number of those LAMP2A receptors.
“CA restores LAMP2A to youthful levels, enabling CMA to get rid of tau and other defective proteins so they can’t form those toxic protein clumps,” said Cuervo.
The researchers tested CA in two different mouse models of Alzheimer’s disease. In both, oral doses of CA administered over four to six months led to improvements in memory, depression and anxiety that made the treated animals resemble or closely resemble healthy, control mice. Walking ability significantly improved in the animal model in which it was a problem and in brain neurons of both animal models, the drug significantly reduced levels of tau protein and protein clumps compared with untreated animals.
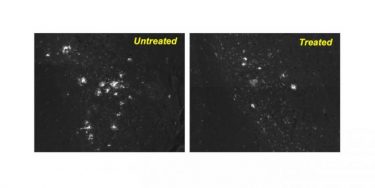

After a mouse model of Alzheimer’s was treated with the drug CA, many fewer protein clumps were present in its brain neurons compared with the neurons in the brain of an untreated mouse [credit: Albert Einstein College of Medicine].
“Importantly, animals in both models were already showing symptoms of disease and their neurons were clogged with toxic proteins before the drugs were administered,” said Cuervo. “This means that the drug may help preserve neuron function even in the later stages of disease. We were also very excited that the drug significantly reduced gliosis – the inflammation and scarring of cells surrounding brain neurons. Gliosis is associated with toxic proteins and is known to play a major role in perpetuating and worsening neurodegenerative diseases.”
Treatment with CA did not appear to harm other organs even when given daily for extended periods of time.
The study was published in Cell.
Related topics
Drug Development, Drug Discovery, In Vivo, Molecular Targets, Neurosciences, Pharmacology, Translational Science
Related conditions
Alzheimer’s disease
Related organisations
Albert Einstein College of Medicine
Related people
Dr Ana Maria Cuervo