GRP78 molecular chaperone revealed as drug target for COVID-19
Posted: 25 May 2021 | Victoria Rees (Drug Target Review) | No comments yet
Using computer modelling, a team has shown that a molecular chaperone called GRP78 could be targeted by drugs in strategies to treat COVID-19.

A new University of Southern California (USC), US, study has revealed how therapies targeting a molecular chaperone called GRP78 might offer additional protection against COVID-19 and other coronaviruses that emerge in the future.
According to the scientists, chaperones like GRP78 are molecules that help regulate the correct folding of proteins, especially when a cell is under stress. However, in some cases, viruses can hijack these chaperones to infect target cells, where they reproduce and spread. GRP78 has been implicated in the spread of other serious viruses, such as Ebola and Zika.
While studies have shown that SARS-CoV-2 infects cells by binding with angiotensin converting enzyme 2 (ACE2) receptors on their surface, the researchers examined whether GRP78 has a role as well.
They found that GRP78 serves as a co-receptor and stabilising agent between ACE2 and SARS-CoV-2, enhancing recognition of the virus’ Spike (S) protein and allowing more efficient viral entry into host cells.
The team say this study provides the first experimental evidence in support of computer modelling predictions, demonstrating that GRP78 binds the S protein of SARS-CoV-2 in cells. The computer modelling also shows that COVID-19 variants that are more infectious bind stronger to GRP78.
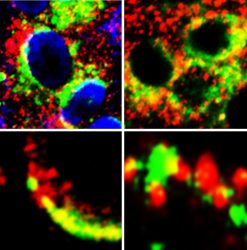

Images of cells showing close proximity (yellow) of GRP78 (green) with S protein of SARS-Cov-2 (red) (left panels) and ACE2 (red) (right panels) [credit: Lead author Dr Anthony Carlos].
Additionally, the researchers found that GRP78 also binds to and acts as a regulator of ACE2 – bringing the protein to the cell surface, which offers SARS-CoV-2 more points to bind with and infect cells.
“Our study reveals that therapy targeting GRP78 could be more effective at protecting and treating people who contract COVID-19 than vaccines alone, particularly when it comes to people who cannot get the vaccine and variants that could bypass vaccine protection but still depend on GRP78 for entry and production,” said senior author Dr Amy Lee.
To investigate the role of GRP78 in SARS-CoV-2 infection, the researchers treated lung epithelial cells with a humanised monoclonal antibody (hMAb159), known to remove GRP78 from the cell surface with no adverse effects in mouse models. Intervention removed GRP78 and reduced ACE2 on the cell surface, diminishing the number of targets to which SARS-CoV-2 could attach.
These findings led the scientists to conclude that interventions, such as hMAb159, to remove cell surface GRP78 could reduce SARS-CoV-2 infection and inhibit the spread and severity of COVID-19 in people who are infected.
While this study used a monoclonal antibody, researchers say there are other agents that could be used to reduce the amount or activity of GRP78, creating multiple pathways for potential drug solutions to target GRP78.
“What is particularly exciting for this finding is that GRP78 could be a universal target in combination with existing therapies not just to combat COVID-19, but other deadly viruses that depend on GRP78 for their infectivity as well,” said Lee.
The next step for the research team is to explore these findings further through animal studies.
The findings are published in the Journal of Biological Chemistry.
Related topics
Antibodies, Bioinformatics, Informatics, Molecular Modelling, Molecular Targets, Target Validation
Related conditions
Covid-19, Ebola virus, Zika virus
Related organisations
University of Southern California (USC)
Related people
Dr Amy Lee