Licensed drug may reduce COVID-19 infection by 70 percent
Posted: 6 August 2021 | Anna Begley (Drug Target Review) | No comments yet
Fenofibrate and its active form, fenofibric acid, have been shown to significantly reduce COVID-19 infection in human cells.
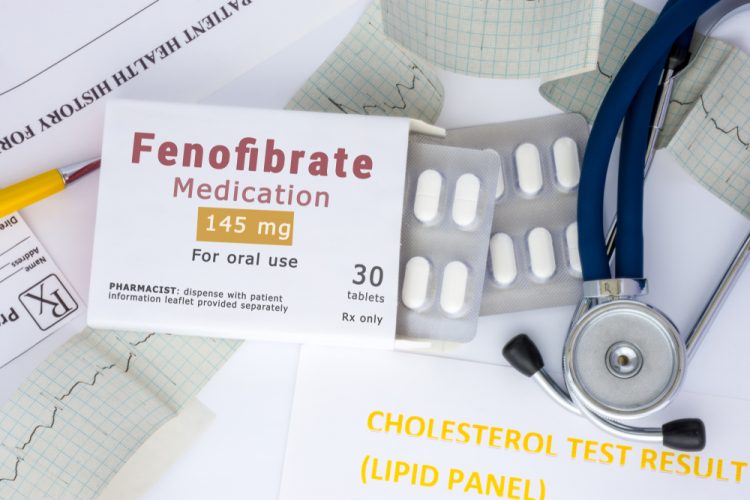

A research team led by the Universities of Birmingham and Keele, both UK, and the San Raffaele Scientific Institute, Italy, have demonstrated that fenofibrate and its active form, fenofibric acid, significantly reduced COVID-19 infection in human cells in the laboratory. Specifically, the licensed drug, which is normally used to treat abnormal levels of fatty substances in the blood, could reduce SARS-CoV-2 infection by up to 70 percent.
SARS-CoV-2 infects the host through an interaction between the Spike (S) protein on the surface of the virus and the ACE2 receptor protein on host cells. The team tested a panel of already licensed drugs, including fenofibrate, to identify candidates that disrupt the ACE2 and S protein interactions. After identifying fenofibrate as a candidate, they then tested the efficacy of the drug in reducing infection in cells in the laboratory using the original strains of the SARS-CoV-2 virus isolated in 2020.
They found fenofibrate reduced infection by up to 70 percent. Additional unpublished data also indicated that fenofibrate is equally effective against the newer variants of SARS-CoV-2, including the alpha and beta variants and research into its efficacy in the delta variant is ongoing. Importantly, reduction of infection was obtained using concentrations of the drug which are safe and achievable using the standard clinical dose of fenofibrate.
“Our data indicates that fenofibrate may have the potential to reduce the severity of COVID-19 symptoms and also virus spread,” said co-author Dr Elisa Vicenzi. “Given that fenofibrate is an oral drug which is very cheap and available worldwide, together with its extensive history of clinical use and its good safety profile, our data has global implications, especially in low-middle income countries and in those individuals for whom vaccines are not recommended or suitable such as children, those with hyper-immune disorders and those using immune-suppressants.”
The team is now calling for clinical trials to test the drug in hospitalised COVID-19 patients, to be carried out in addition to two clinical trials also currently underway in such patients in research being led by the Hospital of the University of Pennsylvania in the US and Hebrew University of Jerusalem in Israel.
The study was published in Frontiers in Pharmacology.
Related topics
Cell Cultures, Drug Delivery, Drug Repurposing, In Vitro, Pharmacology, Protein, Screening, Target Molecule, Targets
Related conditions
Covid-19
Related organisations
Birmingham University, Keele University, San Raffaele Scientific Institute
Related people
Dr Elisa Vicenzi