Polyphor receives CHF 40 million private financing for antibiotic research
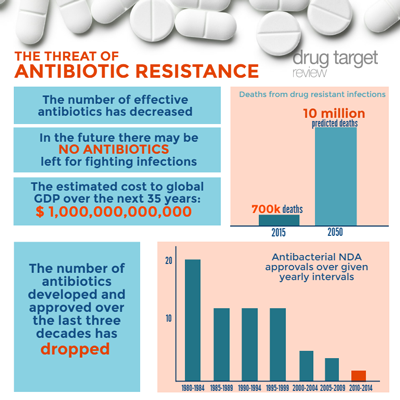
Polyphor Ltd, a clinical stage, privately held Swiss specialty pharma company focused on the development of macrocycle drugs addressing antibiotic resistance and respiratory diseases, has completed a CHF 40 million private placement. Exisiting Polyphor investors contributed to 98% of the financing.